how do hot air balloons work gas laws
The name hot air balloon helps give you some idea of exactly how the propane is used to fly the balloon. The balloon has a fixed volume but there is a hole at the bottom.
ρ PMRT where M is the molar mass of the gas The molar mass of air is about 29 gmol.

. Essentially hot air is lighter than cool air because it has less mass per unit of volume. The balloon thus rises. Sometimes if you think of it in this equation it is a little easier to understand.
When the air inside the envelope is heated the entire balloon begins to rise. P R T P M where M is the molar mass of the gas The molar mass of air is about 29 gmol. V T This means that when the gas in a hot air balloon is heated the gas expands.
So the extra volume flows out of the hole in the bottom of the balloon. One may reasonably ask how do hot air balloons work. There are a few reasons propane is the gas of choice in a hot air balloon.
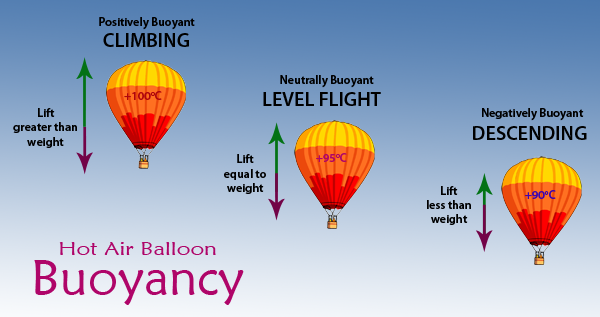
When you fill the balloon with hot air it will eventually fill up all the way. 2 When the air inside is a bit hotter the balloon floats at a steady height because the lift force and weight are now the same. 1 A hot air balloon stays on the ground or descends when the air inside it is too cool.
In this picture it explains why the. There are three basic ways of getting the hot air into a hot air balloon. Using Prezi Video for virtual sales presentations that convert.
Ever since the third century BC it has been known that an object floats when it weighs less. Hot air balloons are based on a very basic scientific principle. When the density of the balloon decreases to be less than the density of the outside air the.
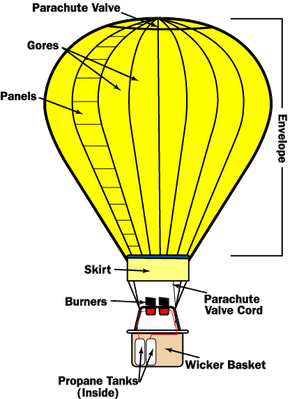
Less pressure means more volume. The envelope the basket and the burners. The reason we know that the hot air is less dense than cool air is due to the Ideal Gas Law.
Gas laws and how hot air balloons work Chapter 5 covers gas lawshow are they relevant to how hot air balloons work Gas laws and sports how to throw a curve ball how do gasses impact the flight of a football soccer ball baseball which is a pitchers versus hitters park and way with respect to gasses and gas laws etc. A hot air balloon is a balloon used for travel through the air in a basket suspended below a large bag of heated air. - A hot air balloon works with the Charles Law.
Boyles Law states that if the temperature remains the same and the pressure changes the volume of the gas in the balloons will decrease as pressure is increased and will increase as pressure is decreased. When the burners are turned on they heat the air inside the envelope. If you heat that air by 100 degrees F it weighs about 7 grams less.
In order to make a hot air balloon rise heat is added to the air inside the balloon. The first way known as the Montgolfier method uses fire to raise the temperature of the air in the balloon. In this case the weight of the balloon blue arrow is greater than the lift red arrow.
Note that the volume of the balloon practically does not change during heating so the displaced air mass or the associated buoyant force does not change either. We can rearrange the Ideal Gas Law PV nRT to calculate the density ρ of the hot air. Warmer air rises in cooler air.
As the air in the balloon is heated by a small flame the balloon expands becoming less dense than the surrounding cool air. Unlike a party balloon a hot air balloon is not a closed system but open at the bottom where the gas burner heats the air and also open at the top more about this later. If the balloon were sealed pressure would soon build to the bursting point.
-Now when you keep heating the air and you raise the temperature this means that the volume will have to increase as well. Just as an object less dense than water rises to. As hot air balloons rise through the sky the pressure decreases this allows the volume of the hot air balloon to increase.
If gas expands when it is heated a given weight of hot air occupies a larger volume then the same weight. This relationship between the temperature and volume of a gas which became known as Charles law provides an explanation of how hot-air balloons work. A law of physics says that hot air rises.
How do air bags in a car work. How to schedule fewer meetings and get more done. As a result of his work with balloons Charles noticed that the volume of a gas is directly proportional to its temperature.
We know from the ideal gas laws that when you heat a gas under constant pressure it will expand. The Ideal Gas Law states that the Pressure times the Volume is equal to the number of molecules times the gas constant R times the Temperature. Since there is less air in the same.
This relationship between the temperature and volume of a gas which became known as Charles law provides an explanation of how hot-air balloons work. Boyles Law can be used to describe why a hot air balloon is able to keep such a large volume in the sky. As air inside the balloon heats up the molecules move faster and faster.
The balloon has a fixed volume so the extra volume flows out of the hole in the bottom of the balloon. Charles Law says that the volume of a gas is directly proportional to the temperature of the gas. It is used for recreational activities but many scientific principles apply to it.
The hot air balloon operates on the principle of Charless Law which states that the volume of a gas increases with temperature. To land a valve on top of the hot air balloon is opened that allows the hot air to. This says that the density of the air decreases as itse temperature increases.
We can rearrange the Ideal Gas Law PVnRT to calculate the density p of the hot air. - The gas that is released into the bag is hot due to the reaction causing the bag to fill or inflate - Since the gas is hot it expands rapidly filling the airbag quickly. This being Boyles Law.
In a hot air balloon the air that is heated is not in a rigid container but in a balloon that. Before long the hot air inside the balloon is less dense than the cool air that surrounds it. The higher the temperature gets the more the volume of the gas in a balloon will increase.
- As you keep heating the air it gets bigger and since the balloon can only hold so much air it starts to let out small amounts of air. Hot Air Balloons By Bhaavi Patel What is a hot air balloon. Is a hot air balloon Charles Law.
Fill a balloon with hot air let it go and it will rise up in the air. - However this reaction is also connected to the Charles Law. Many people think hot air balloons are using helium because a typical birthday balloon is often filled with helium.
But molecules are free to escape. Thus the heated air inside the balloon is less dense than the cool air outside of the balloon. How does Boyles law relate to hot air balloons.
This is the type of balloon used by most hot air balloon pleasure flights. How to get repeat customers. A cubic foot of air weighs roughly 28 grams about an ounce.
Hot air balloons are made up of three primary parts. How Hot Air Balloons Work The law that explains how hot air balloons work is the Charless Law.

Gas Laws Hot Air Balloons By Jonathan Dauterman
Section 2 Gas Behavior Objective What Gas Law Explains Why This Ppt Video Online Download

Charles Law And Gay Lussac S Law Let S Talk Science

Ps1 7 Gas Laws Charles S Law Youtube

Charles Law And Gay Lussac S Law Let S Talk Science

Combined Gas Law Ck 12 Foundation

How Hot Air Balloons Work Gas Laws